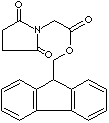
PRODUCT IDENTIFICATION
H.S. CODE
2925.19
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white powder
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions. Moisture sensitive.
GENERAL DESCRIPTION & APPLICATIONS
Urethanes Protecting Groups: Urethanes find extensive use as protecting groups for amines. These protecting groups are easily formed (see below) and can be readily removed using the appropriate conditions. Several very popular urethane protecting groups have been developed that withstand a variety of harsh reaction conditions but are readily removed when subjected to mild acid, hydrogenolysis or mild basic media. The different cleavage conditions of the above urethane protecting groups has enabled so-called ��orthogonal�� protection strategies to be developed that enable selective chemistries to be performed upon different amines present in the same molecule (http://users.ox.ac.uk/)
|
|
|
|
APPEARANCE
white to off-white powder
IDENTITY [IR[
pass
PURITY (HPLC)
98.0% min
PRICE INFORMATION